Abstract
Background Paroxysmal nocturnal haemoglobinuria (PNH) is a haematopoietic stem cell disorder in which an acquired somatic mutation of the PIGA gene in a haematopoietic stem cell results in the deficiency of key complement regulatory molecules, namely CD59 and CD55, from the surface of blood cells leading to an increased susceptibility of blood cells to complement-mediated activation +/- intravascular haemolysis and thrombosis. Thrombosis has been the leading cause of death in PNH. Multiple mechanisms of thrombosis in PNH have been proposed, including cross talk between the coagulation and complement system. This may lead to denser clots which are more resistant to fibrinolysis. Treatment with eculizumab, which inhibits complement factor C5 and thereby prevents terminal complement activation, reduces thromboembolic events in PNH, indicating that complement activation plays a significant role in the pathophysiology of thrombosis in this disease. Patients with other thrombotic disorders are known to form denser fibrin clots that incur greater resistance to fibrinolysis. It has not previously been explored whether changes to clot architecture contribute to thrombosis in PNH. Furthermore, the role of eculizumab in clot architecture is undocumented.
Aims Characterise fibrin clot structure in patients with PNH and determine the effect of eculizumab on clot structure.
Methods Informed consent was obtained from all participants. 53 patients from the PNH National Service (Leeds) were recruited. They were grouped according to the proportion of PNH granulocytes, <40% (n=18), 40> - < 90 %(n=18) and >90% (n=17) and reanalysed according to treatment with (n=19) or without (n=32) eculizumab. Plasma samples were obtained and ex vivo fibrin clot structure and function was analysed by permeation analysis of clot pore structure, turbidity analysis of clot formation and fibrinolysis, and fibrin ultrastructure by laser scanning confocal microscopy and scanning electron microscopy, according to methodology and protocols well established in our laboratory. The proportion of PNH granulocytes was measured by flow cytometry and fibrinogen levels by Clauss. P values of <0.05 were taken to indicate significance.
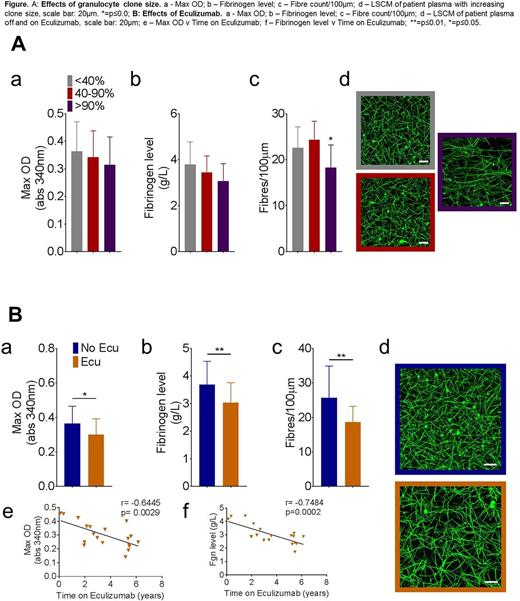
Results The proportion of PNH granulocytes correlated with fibrinogen (r= -0.35, p=0.048), maximum absorbency (r= -0.34, p=0.046) and clot density (r= -0.36, p=0.04). Seventy per cent of patients with large proportions of PNH granulocytes were on eculizumab. Analysis of patients not on eculizumab showed a trend towards proportion of PNH granulocytes correlating positively with fibrinogen levels (r=0.42, p=ns). Patients on eculizumab had lower fibrinogen levels (3.1±0.2 v 3.6±0.1g/L, P=0.02), maximum absorbance (0.31±0.1 v 0.40±0.1, p=0.028) and fibre density/100µm (18.5±4.4 v 23.9±4.2, p=0.006), indicative of a less dense clot.
Summary/Conclusions This is the first study to demonstrate that patients on eculizumab showed reduced fibrinogen, fibre thickness and fibre density. Our data indicate that the beneficial effects of eculizumab in PNH is partly due to reduced complement activation and attenuated inflammatory response, leading to lower fibrinogen levels and a less thrombotic fibrin clot structure. These novel data help to explain the impact of complement inhibition in this disease.
Arnold: Alexion Pharmaceuticals, Inc.: Honoraria. Griffin: Alexion Pharmaceuticals, Inc.: Honoraria. Munir: Janssen: Honoraria; Alexion Pharmaceuticals, Inc.: Honoraria; Roche: Honoraria; AbbVie: Honoraria; Gilled: Honoraria. Hillmen: Gilead: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding; Alexion Pharmaceuticals, Inc.: Consultancy, Honoraria; Pharmacyclics LLC, an AbbVie Company: Honoraria, Research Funding; Celgene: Research Funding; AbbVie: Consultancy, Honoraria, Research Funding; Roche: Consultancy, Honoraria, Research Funding; Novartis: Honoraria, Research Funding; GSK: Consultancy, Honoraria, Research Funding. Richards: Alexion Pharmaceuticals, Inc.: Honoraria. Hill: Alexion Pharmaceuticals, Inc.: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.